The Power Within: Understanding the Basics of Batteries
Unlocking the Secrets Behind the Power Sources of Our Modern World
INTRODUCTION
In our fast-paced, technology-driven world, batteries play a pivotal role in powering our daily lives. From the smartphone in your pocket to the electric vehicle on the road, batteries are the silent workhorses that keep everything running. But have you ever wondered what goes into making a battery? How do they work? What are the different types, and what does the future hold for this essential technology? Let’s delve into the world of batteries and uncover the power within.
The Anatomy of a Battery
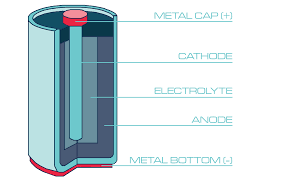
At its core, a battery is a device that stores chemical energy and converts it into electrical energy when needed. This process involves three main components:
- Anode (Negative Electrode): This is where oxidation occurs, meaning it loses electrons during the discharge process.
- Cathode (Positive Electrode): This is where reduction occurs, meaning it gains electrons during the discharge process.
- Electrolyte: A medium that allows the flow of electrical charge between the cathode and anode.
When a battery is in use, electrons flow from the anode to the cathode through an external circuit, powering your device. This flow of electrons generates a current, which is what we harness to run electronic devices.
Types of Batteries
Batteries come in various shapes, sizes, and chemistries, each suited for different applications. Here are the most common types:
1. Primary Batteries
These are single-use batteries that cannot be recharged. They are commonly found in everyday items like remote controls, toys, and flashlights. The most popular primary batteries include:
- Alkaline Batteries:

primary batteries Known for their long shelf life and high energy density, these are the most commonly used primary batteries.
- Zinc-Carbon Batteries: These are more affordable but have a shorter lifespan and lower energy density compared to alkaline batteries.https://www.tycorun.com/blogs/news/the-ultimate-comparative-analysis-of-carbon-zinc-battery-vs-alkaline-which-is-better
- Lithium Batteries: Lightweight and with a high energy density, lithium batteries are used in high-performance devices like cameras and medical equipment.
2. Secondary Batteries

These are rechargeable batteries, designed for repeated use. They are integral to many modern technologies, including smartphones, laptops, and electric vehicles. Common secondary batteries include:
- Lithium-Ion Batteries: These are widely used due to their high energy density, lightweight, and long cycle life. They are found in almost all modern portable electronics and electric vehicles.
- Nickel-Cadmium (NiCd) Batteries: Known for their robustness and ability to deliver high discharge rates, they are used in power tools and emergency lighting.
- Nickel-Metal Hydride (NiMH) Batteries: These are an eco-friendly alternative to NiCd batteries, offering a higher capacity and longer life without the toxic cadmium.
- Lead-Acid Batteries: Primarily used in automotive applications and backup power supplies, these batteries are known for their reliability and ability to deliver high surge currents.
How Batteries Work
The operation of a battery is a chemical reaction known as a redox (reduction-oxidation) reaction. Here’s a simplified breakdown of the process:
- Discharging: When a battery powers a device, a chemical reaction occurs in the anode that releases electrons. These electrons flow through the external circuit to the cathode, creating an electric current that powers the device. Meanwhile, ions in the electrolyte move from the anode to the cathode to balance the charge.
- Charging (for rechargeable batteries): When recharging, the process is reversed. An external power source applies a voltage to the battery, forcing electrons to flow back to the anode, restoring the battery’s chemical potential.https://www.google.com/imgres?q=how%20battery%20works%20video&imgurl=https%3A%2F%2Fi.ytimg.com%2Fvi%2FgWKOjncBMCQ%2Fmaxresdefault.jpg&imgrefurl=https%3A%2F%2Fwww.youtube.com%2Fwatch%3Fv%3DgWKOjncBMCQ&docid=lclaaZOccU8scM&tbnid=PPKb7UW1hTBmmM&vet=12ahUKEwii66KOoIiHAxUobEEAHQ-2DIYQM3oECBQQAA..i&w=1280&h=720&hcb=2&ved=2ahUKEwii66KOoIiHAxUobEEAHQ-2DIYQM3oECBQQAA
Innovations and the Future of Batteries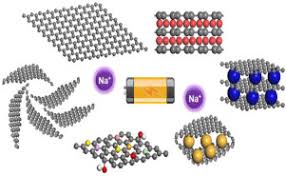
As technology advances, the demand for better, more efficient batteries is ever-increasing. Several promising developments are on the horizon:
- Solid-State Batteries: These use a solid electrolyte instead of a liquid one, offering higher energy density, faster charging times, and improved safety.
- Sodium-Ion Batteries: A potential alternative to lithium-ion batteries, sodium-ion batteries use abundant sodium resources, making them more cost-effective and environmentally friendly.
- Graphene Batteries: Leveraging the remarkable properties of graphene, these batteries promise ultra-fast charging, higher capacities, and longer lifespans.
- Flexible Batteries: These batteries are designed for wearable electronics and flexible devices, paving the way for innovative applications in medical devices and smart textiles.
Environmental Impact and Recycling
While batteries are indispensable, they also pose environmental challenges. The production and disposal of batteries can lead to pollution and resource depletion. Therefore, recycling batteries and developing sustainable materials are critical.
- Recycling Programs: Many countries have implemented battery recycling programs to recover valuable materials and reduce environmental impact. Consumers are encouraged to dispose of batteries properly at designated recycling centers.
- Sustainable Materials: Research is ongoing to develop batteries using sustainable and abundant materials, reducing the dependency on rare and toxic elements like cobalt and lead.
Conclusion
Batteries are the unsung heroes of our modern world, powering everything from the smallest gadgets to the largest electric vehicles. Understanding their inner workings, the different types available, and the future trends in battery technology helps us appreciate the power within. As we move towards a more electrified and sustainable future, innovations in battery technology will continue to play a crucial role, ensuring we stay powered up and connected.



